The Food and Drug Administration (FDA) has granted
authorization for the use of the first artificial intelligence-powered medical
device designed to assist doctors in detecting common forms of skin cancer. Developed
by DermaSensor, a Miami-based medical device company, the technology does not
serve as a screening tool but aids in the evaluation of lesions already
identified as suspicious by physicians.
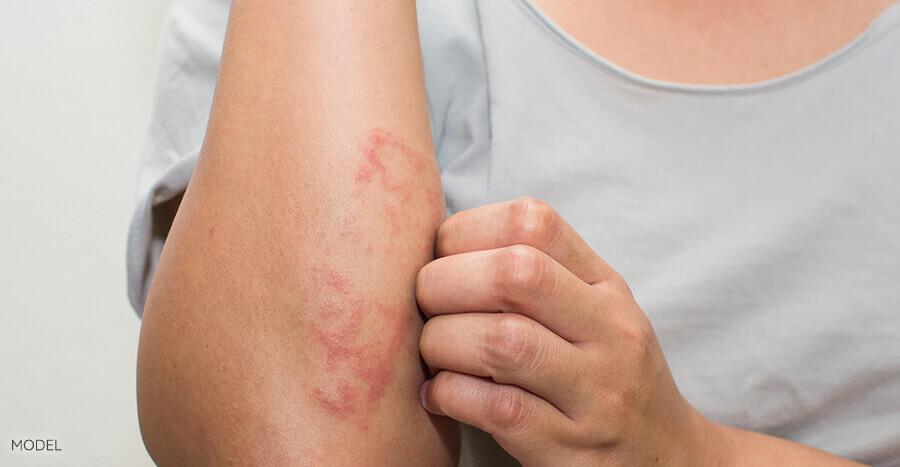
The handheld device employs AI-powered spectroscopy to analyze
cellular and subcutaneous characteristics of lesions. Known as DermaSensor, the
device utilizes a proprietary algorithm trained on data from over 4,000
malignant and benign lesions, providing real-time results. A "spectral
similarity score" is delivered to complement a physician's assessment,
offering a high-tech method beyond visual inspection.
Designed for use by primary care physicians and
dermatologists, DermaSensor caters to patients aged 40 and above. The device
follows a simple process: a doctor identifies a suspicious lesion, the wireless
device records and scans it, and the algorithm delivers an immediate
assessment. Results include "Investigate Further" for specialist
examination and "Monitor" for cases not requiring immediate
evaluation.
Beyond detecting melanoma, the deadliest skin cancer,
DermaSensor assesses moles for basal cell carcinoma and squamous cell
carcinoma. With one in five Americans developing skin cancer by age 70, early
detection is crucial. The FDA mandates additional validation testing across
diverse demographic groups to enhance the device's reliability and
effectiveness. As AI continues to reshape healthcare, DermaSensor represents a
milestone in leveraging technology for proactive skin cancer detection and care
optimization.






.jpg)